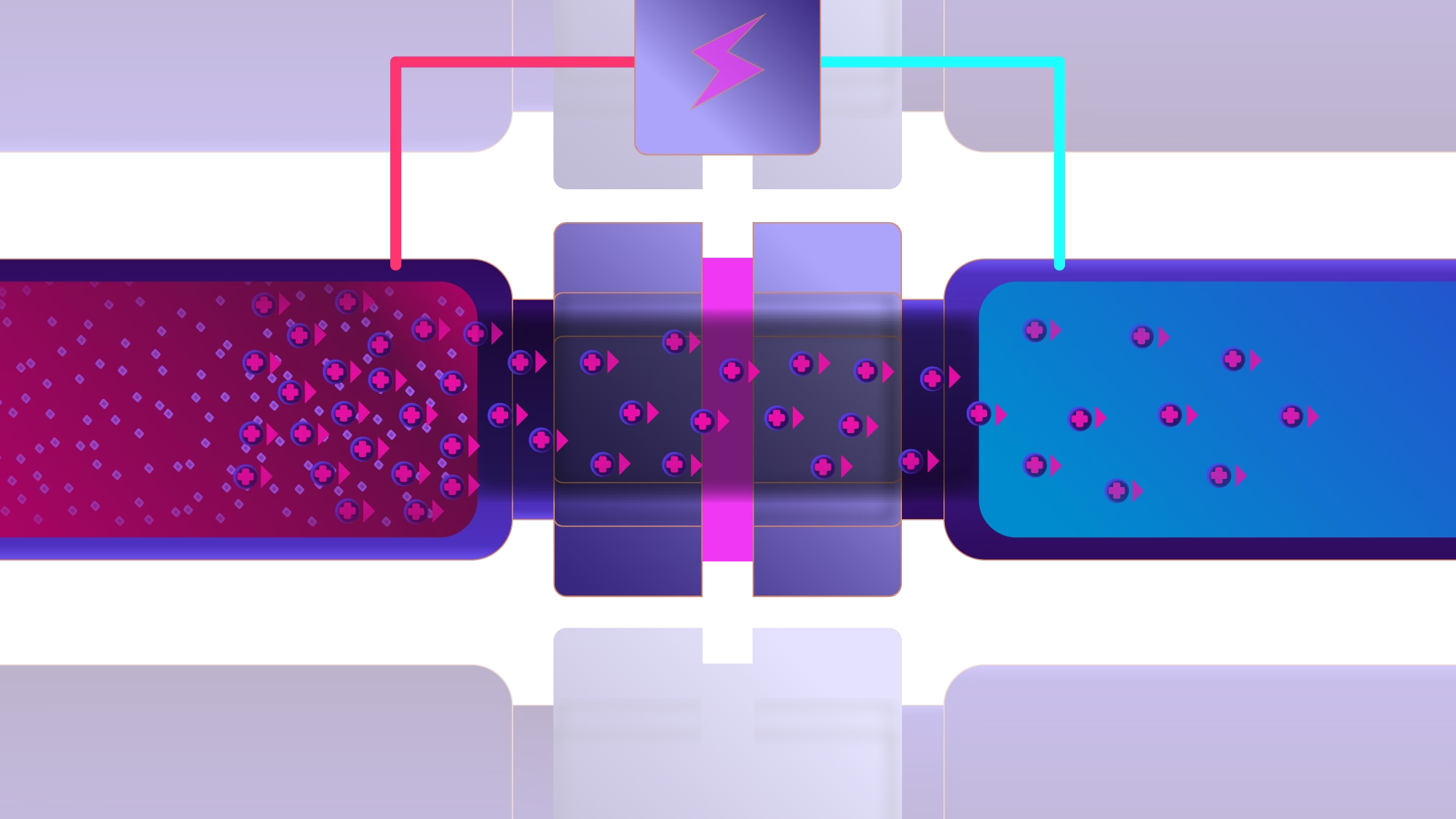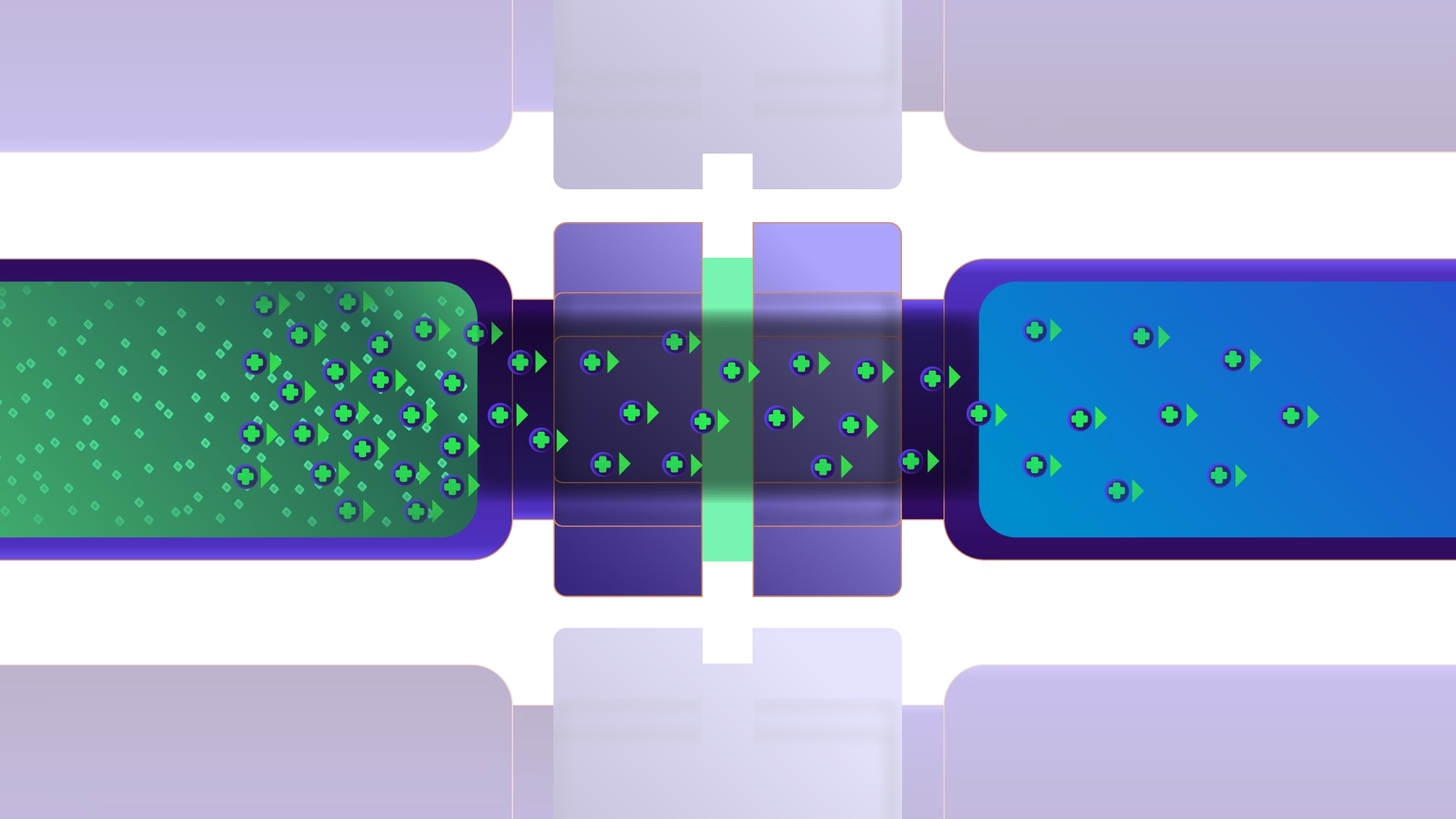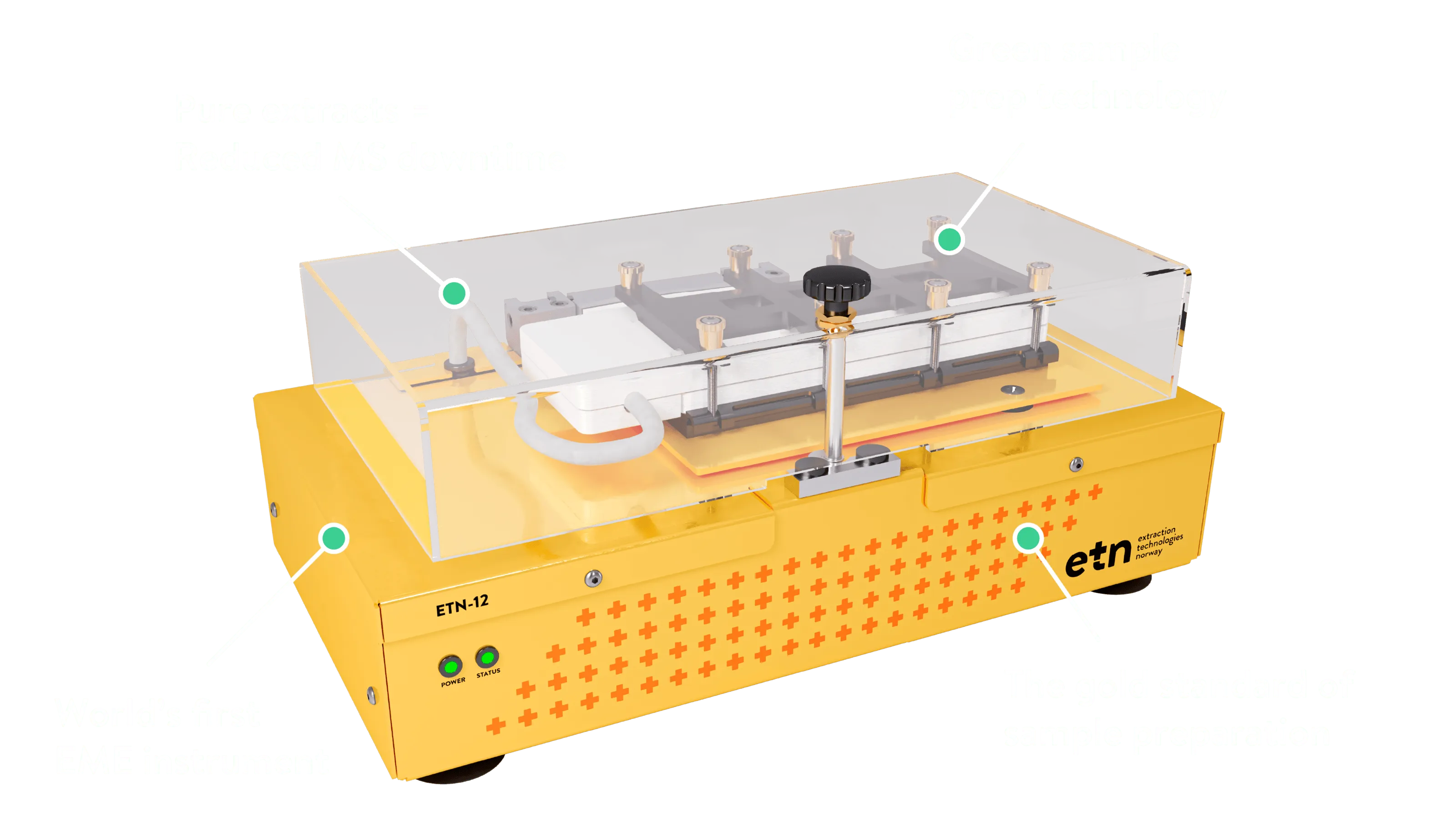
Both are non-destructive, give pure sample extracts, are environmentally friendly and have the possibility of enrichment. Both techniques use a similar setup with an aqueous sample and an acceptor solution in separate vials, separated by a thin organic solvent layer known as the supported liquid membrane (SLM).