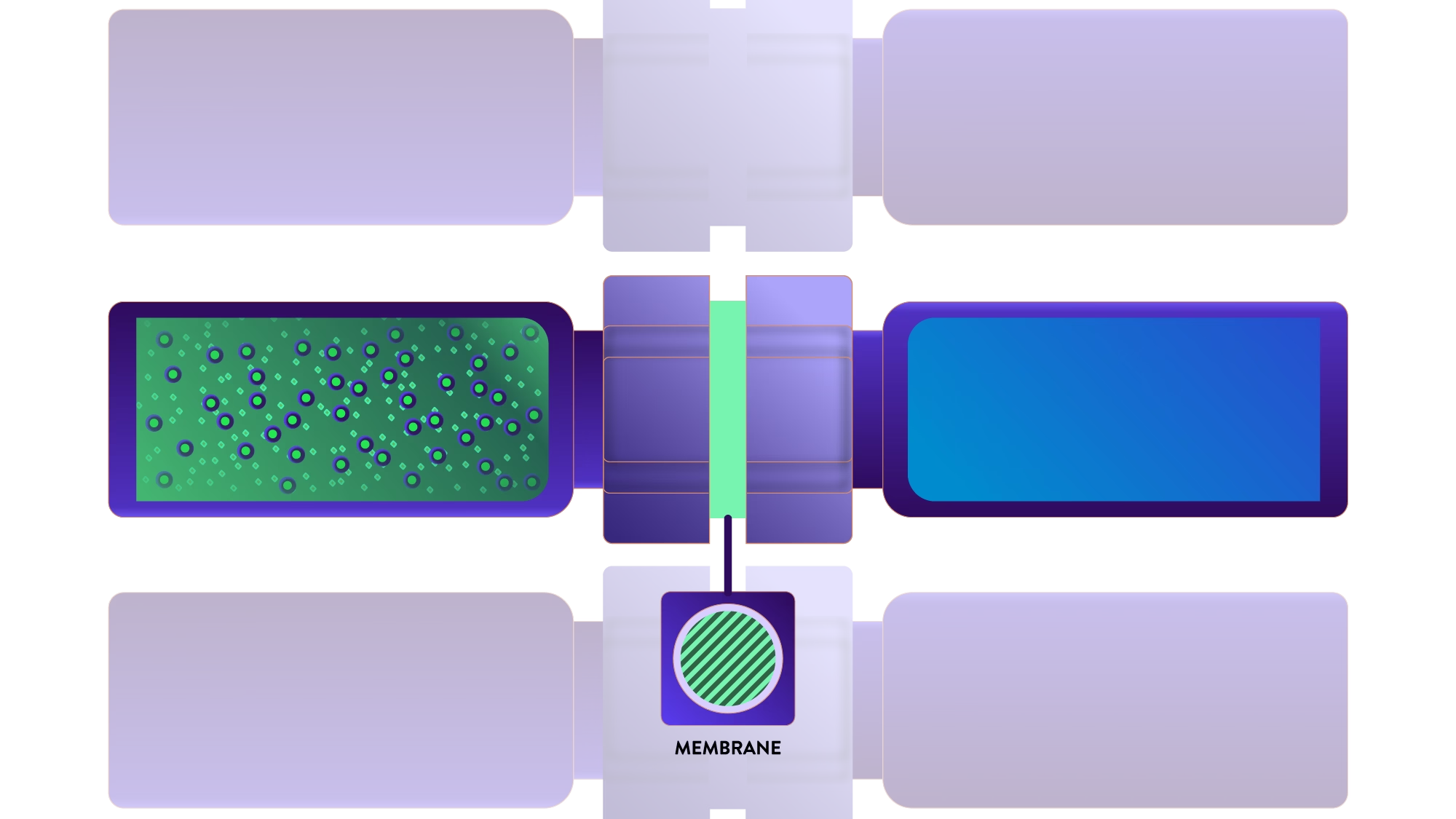
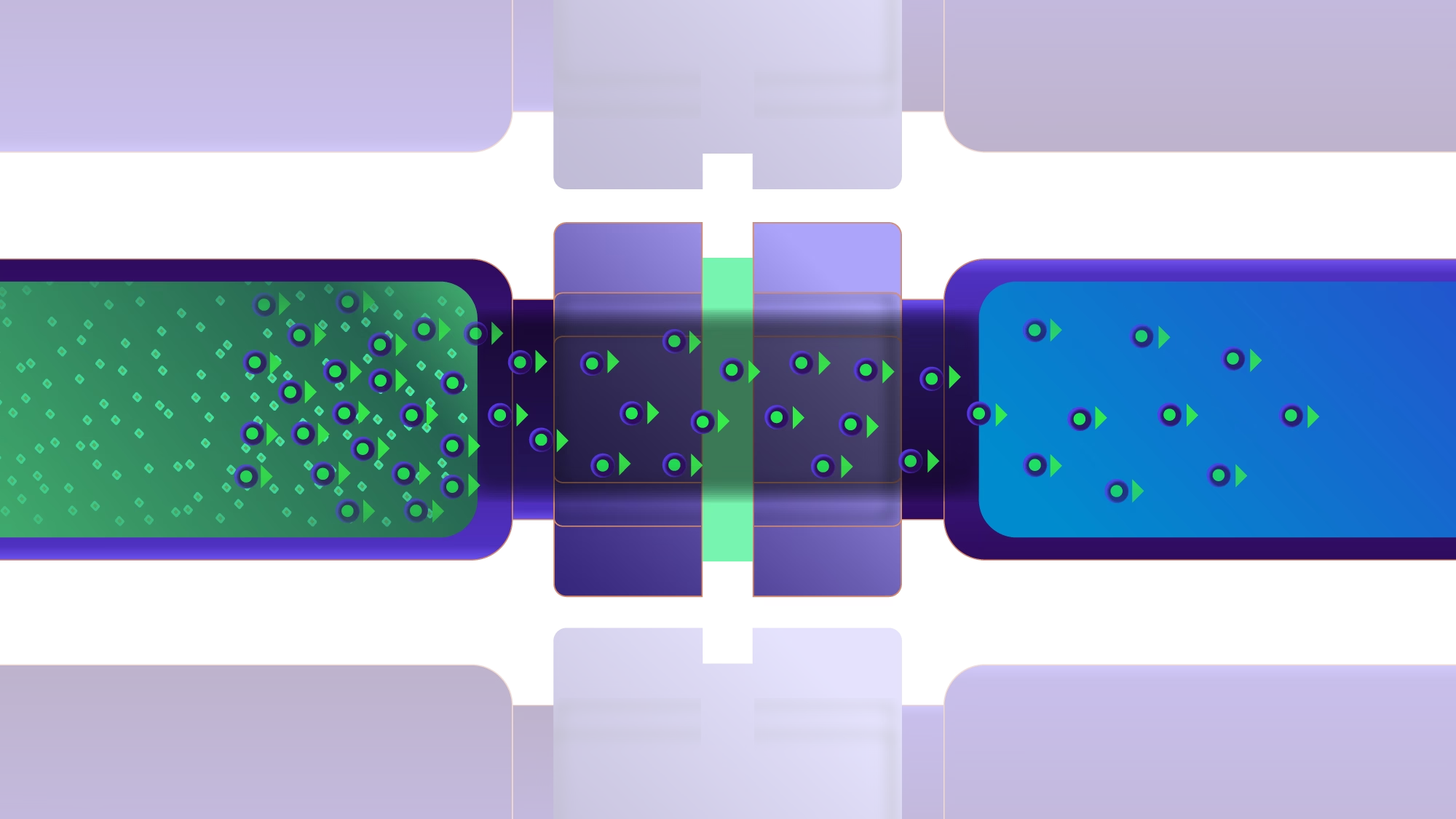
Extraction of very weak acids, bases, and un-ionizable compounds
<Mer tekst kommer>
Minimal Solvent Usage and Environmental Impact
LME offers a significant reduction in solvent consumption compared to traditional methods such as SPE and LLE. In SPE, large volumes of organic solvents are often required to elute analytes from the sorbent material, leading to considerable solvent waste and environmental impact. Similarly, LLE involves the use of two immiscible liquid phases, further exacerbating solvent usage. In contrast, LME operates with minimal solvent volumes, typically in the microliter range, thereby reducing solvent waste and aligning with principles of green analytical chemistry.
Compatibility with Small Sample Volumes
LME offers compatibility with small sample volumes, making it ideal for applications where sample availability is limited. In contrast, traditional methods such as SPE and LLE may necessitate larger sample volumes to achieve sufficient analyte recovery. By requiring only a small volume of sample, LME reduces sample consumption and facilitates the analysis of precious or scarce samples, particularly in fields such as clinical diagnostics and forensic analysis.
LME has the potential to become responsible for the paradigm shift because the technology provides:
- Excellent reproducibility
- Pure extracts
- Green sample preparation method